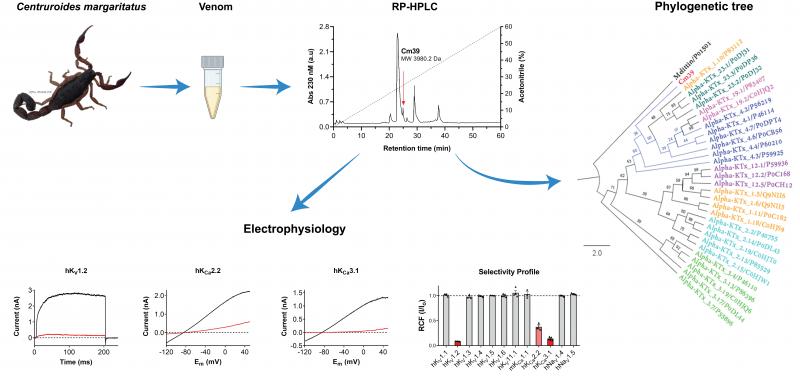
Novel peptide toxin that inhibits Kv1.2 and Ca2+-activated potassium channels KCa2.2 and KCa3.1
Muhammad Umair Naseem, Gyorgy Panyi and his colleagues in international collaboration with Prof. Possani’s research group, UNAM, Mexico work on peptide toxins that inhibit different potassium channels and hold potential to be used as therapeutic drugs. Here, the team studied a novel scorpion toxin isolated from the venom of Centruroides margaritatus, Cm39. It is a 37 amino acids long peptide which has high similarity with already known K+ channel inhibitor scorpion toxin (KTx) and phylogenetic analysis proposed that Cm39 belongs α-KTx 4 family. Cm39 exhibited unusual selectivity profile for various channels tested in this study. Voltage-gated K+ channel Kv1.2 and Ca2+-activated KCa2.2 and KCa3.1 channels are sensitive to this toxin. The blocking affinity of Cm39 for Kv1.2 and KCa3.1 is in low-nanomolar range however, the affinity for KCa2.2 is modest. The conductance–voltage relationship of Kv1.2 and analysis of the toxin binding kinetics revealed that Cm39 follows the pore blocking mechanism. Several other potassium and sodium channels were found insensitive to a high concentration (1 µM) of Cm39. Cm39 may serve as a potential drug candidate which can target both KCa2.2 and KCa3.1 channels simultaneously and thereby helping to reduce the atrial fibrillation. The findings of this study were published in the MDPI journal Toxins. (DOI: https://doi.org/10.3390/toxins15010041)