|
Head of the group email: Webpage: |
|---|
Members of the group
 |
|
Former members of the group
|
Theoretical overview:
Our laboratory focuses on the electrophysiological properties of electrically non-excitable cells. We mainly study the physiological and pathophysiological role of voltage-gated ion channels, with a special emphasis on K+ channels. One of the main goals of our research is the study of the pharmacological, biophysical and cell biology of the ion channels of immune cells. Voltage-gated K+ channels maintain a membrane potential in these cells that allows efficient Ca2+ -dependent signaling pathways for antigen recognition. Among the ion channels, the role of the Kv1.3 K+ channel stands out, but in the last decade our knowledge about the role of other K+, Ca2+, Na+, H+ and other channels has also expanded significantly. Disruption of the physiological activity of Kv1.3 channels may diminish or completely abolish the response of T cells to the antigen, thus, achieving immunosuppression.
Research projects:
1. Molecular pharmacology of T cell ion channels (project leader: Gyorgy Panyi)
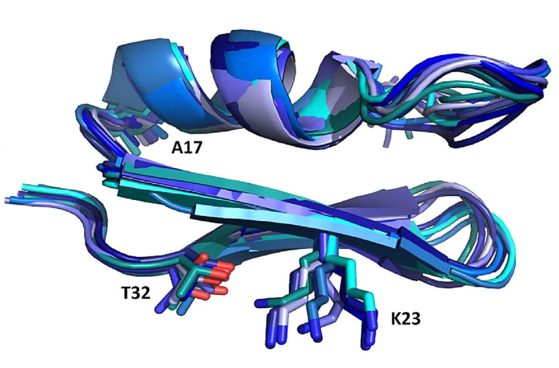 Figure 1: NMR structure of Anuroctoxin containing N17A/F32T substitutions. The figure also shows the K23 amino acid side chain critical for ion channel inhibition. The structure of the doubly substituted toxin is much stiffer than that of the wild type, which is associated with increased Kv1.3 selectivity
|
|
|
Small peptides (30-35 amino acids) isolated from scorpion venom are very potent K+ channel inhibitors (with nM or even pM affinity). Since block of Kv1.3 channels results in inhibition of T cell activation and proliferation, these blockers can serve as a template for the development of high-specificity and affinity Kv1.3 inhibitors that can be used in the therapy of various effector memory T cell (TEM) -related autoimmune diseases. In recent years, we have identified one of the highest affinity Kv1.3 inhibitor, Vm24, which has been isolated from the venom of V. mexicanus smithi (Kd = 3 pM). The toxin inhibits the Kv1.3 channel at least 1,500-fold more effectively than all other ion channels studied and suppresses the delayed-type hypersensitivity response, a model of autoimmune diseases in rats. Our current research aims to increase the specificity and affinity of toxins for the Kv1.3 channel. Using solid-phase synthesis or recombinant techniques wild-type and toxins with substitutions at specific positions are produced. Together with the group of Zoltán Varga, we successfully improved the selectivity of Anuroctoxin for Kv1.3. The N17A/F32T toxin produced in the lab lost its ability to inhibit Kv1.2 channels while retaining high affinity for Kv1.3 (Fig. 1). Currently, analogs of the Vm24 peptide are produced to increase selectivity for Kv1.3. In addition, we design conjugates that provide a specific target delivery of the toxin, e.g. into the central nervous system.
|
2. Distribution of Kv1.3 and CRAC channels in lymphocyte membrane (project leader: Peter Hajdu)
|
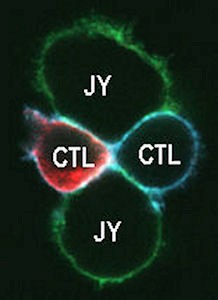
Figure 2
|
|
|
The recruitment of membrane proteins into the signaling platform called “immunological synapse (IS)” plays a key role in the activation of T lymphocytes. We have previously shown that the lateral distribution of Kv1.3 channels in the T cell membrane is not random and the channel colocalizes with the T cell receptor/CD3 complex. Kv1.3 channels are enriched in the IS formed between the cytotoxic T cell and the target cell (Fig. 2). Our present research goal is to identify the physiological consequences of the rearrangement of Kv1.3 and CRAC Ca2+ channels into the IS. We have shown that the recruitment of Kv1.3 channels into the IS is significantly influenced by the interaction of the C-terminal part of the channel with the PSD-95 adapter protein, and we identified the exact role of the HRET sequence in this, which sequence was considered to be responsible for membrane targeting of the Kv1.3 channel. We are currently investigating the molecular interactions that regulate the enrichment of CRAC channels in the IS (e.g. adapter proteins). We also aim at understanding the physiological role of the CRAC recruitment into the IS in the regulation of the Ca2+ signal and the membrane potential.
|
3. Characterization of ion channel expression of immune cells involved in the development of autoimmune diseases and the regulation of immunity against tumors. (project leaders: Gabor Tajti and Peter Hajdu)
Many cell types identified by cell surface and intracellular markers are involved in the development of the immune response. The ion channel expression pattern is unknown for the Th1, Th2, Th17 Treg subgroups of human T cells. Consequently, very little is known about the functional consequences of a specific ion channel expression. In case of autoimmune-based chronic inflammatory bowel disease (IBD, Ulcerative Colitis, and Chron's disease), we have developed the isolation techniques for T cell subtypes from colon biopsy samples and these T cell subsets are currently characterized by patch-clamp. We hope to target specifically the disease-causing T cell subset by inhibiting the ion channels expressed in that subset. In another new research program, we investigate how the immunosuppressive effect of the tumor microenvironment can be reduced by targeting ion channels. To do this, we identify the ion channels expressed in tumor infiltrating lymphocytes (TIL) and immune cells (Treg and Myeloid Derived Suppressor cells) having immunosuppressive properties.
4. Significance of ion channels in glioblastoma and mesenchymal stem cells (project leaders: Gyorgy Panyi and Beata Arnodi-Meszaros).
In addition to regulating electrical excitability, K+ channels also effectively regulate cell division and differentiation. Ion channels have become targets for tumor therapy, as K+ channel inhibitors decrease tumor cell proliferation. A new research program of our group investigates the expression of Ca2+-activated K+ channels and their accessory ß subunits in glioblastoma multiforme cell line and primary patient-derived glioblastoma samples. Our goal is to understand the tumor-specific appearance of Ca2+-activated K+ channel/ß subunit combinations and to target them with small molecule inhibitors developed in the laboratory. Numerous ion channels have also been described in mesenchymal stem cells (MSCs), but the relationship between each differentiation pathway and the function of ion channels is not yet known. We aim to investigate the ion channels involved in the regulation of MSC differentiation and migration. We focus in particular on the voltage-gated proton channel hHv1, the functional expression of which has recently been confirmed in cMSCs in our laboratory.
5. Biophysics of voltage-gated potassium channels (project leader: Tibor Szanto G.)
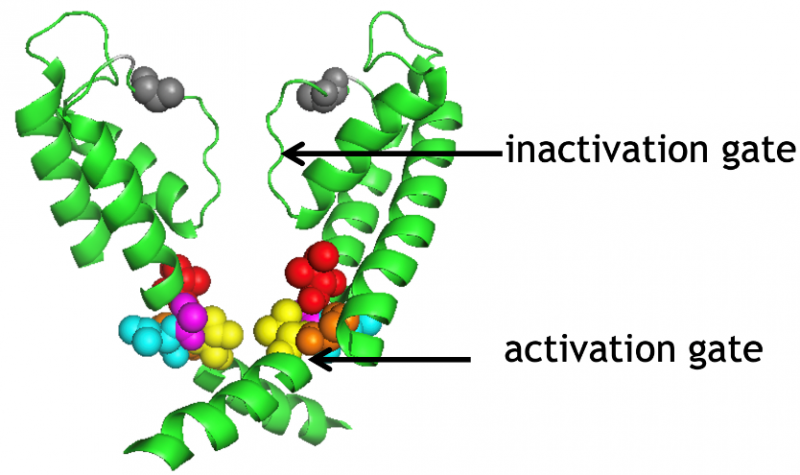 Figure 3
|
|
|
Inactivation of voltage-gated potassium channels limits the K+ conductance available to regulate membrane potential. Using biophysical and molecular biological tools, we showed that the activation and inactivation gates of the voltage-gated Shaker K+ channels are coupled (Fig. 3): the rate of movement of the activation gate depends on the state of the inactivation gate. State-dependent cysteine modification assay demonstrated that the orientation of the 6th transmembrane helix of the channel changes significantly upon inactivation. Using a similar experimental system, we are currently investigating the molecular mechanism of recovery from inactivation and the possibility of inactivation of the channel from the closed state. The role of the hydrogen-bond network, which plays a significant role in the structural stability of the selectivity filter and consequently inactivation, was recently investigated using heavy water (D2O) substitution experiments in Shaker K+ channels.
|
Representative publications:
1. Panyi G, Varga Z, Gaspar R. Ion channels and lymphocyte activation. Immunology letters. 92 (1-2): 55-66., 2004; IF: 2.136, Rank: Q2
2. Panyi G, Vamosi G, Bacso Z, Bagdany M, Bodnar A, Varga Z, Gaspar R, Matyus L, Damjanovich S. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proceedings of the National Academy of Sciences of the United States of America. 101 (5): 1285-90., 2004; IF: 10.452, Rank: D1
3. Panyi G, Deutsch C. Cross talk between activation and slow inactivation gates of Shaker potassium channels. The Journal of general physiology. 128 (5): 547-59., 2006; IF: 4.962, Rank: D1
4. Zsiros E, Kis-Toth K, Hajdu P, Gaspar R, Bielanska J, Felipe A, Rajnavolgyi E, Panyi G. Developmental switch of the expression of ion channels in human dendritic cells. J Immunol. 183 (7): 4483-92, 2009; IF: 5.646, Rank: D1
5. Varga Z, Gurrola-Briones G, Papp F, Rodríguez de la Vega RC, Pedraza-Alva G, Tajhya RB, Gaspar R, Cardenas L, Rosenstein Y, Beeton C, Possani LD, Panyi G. Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol Pharmacol. 82 (3): 372-82., 2012; IF: 4.411, Rank: Q1, D1
Recent publications:
1. Karbat I, Altman-Gueta H, Fine S, Szanto T, Hamer-Rogotner S, Dym O, Frolow F, Gordon D, Panyi G, Gurevitz M, Reuveny E. Pore-modulating toxins exploit inherent slow inactivation to block K(+) channels. Proc Natl Acad Sci U.S.A.; 116 (37):18700-18709., 2019; IF: 9.58, Rank: D1
2. Richards KL, Milligan CJ, Richardson RJ, Jancovski N, Grunnet M, Jacobson LH, Undheim EAB, Mobli M, Chow CY, Herzig V, Csoti A, Panyi G, Reid CA, King GF, Petrou S. Selective Na(V)1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc Natl Acad Sci U.S.A. 115 (34): E8077-E8085., 2018; IF: 9.58, Rank: D1
3. Voros O, Szilagyi O, Balajthy A, Somodi S, Panyi G, Hajdu P. The C-terminal HRET sequence of Kv1.3 regulates gating rather than targeting of Kv1.3 to the plasma membrane. Sci Rep. 8 (5937): 1-14, 2018, IF: 4.011, Rank: D1
4. Fineberg JD, Szanto TG, Panyi G, Covarrubias M. Closed-state inactivation involving an internal gate in Kv4.1 channels modulates pore blockade by intracellular quaternary ammonium ions. Sci Rep. 6 (31131): 1-15., 2016; IF: 4.259, Rank: D1
5. Panyi G, Beeton C, Felipe A. Ion channels and anti-cancer immunity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 369 (1638): 20130106., 2014; IF: 7.055, Rank: D1