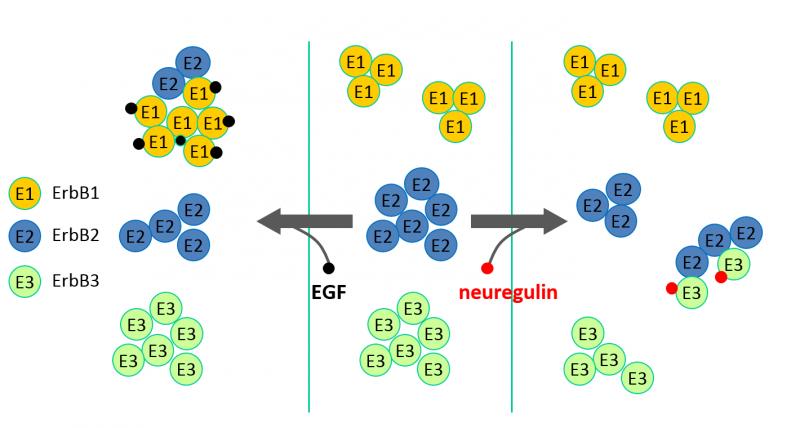
ErbB proteins belong to the epidermal growth factor (EGF) receptor family of receptor tyrosine kinases. The family has four members (ErbB1-4, also known as HER1-4), from which ErbB1, the founding member, is also called EGF receptor. ErbB1 is a receptor for EGF and other EGF-like ligands, whereas ErbB3 and ErbB4 are stimulated by different kinds of neuregulins (NRG, also known as heregulins, HRG). ErbB2 is a co-receptor for the other three members enhancing their signaling potency. According to the accepted theory, developed mainly for ErbB1, unstimulated, monomeric receptors dimerize upon growth factor binding, whose primary driving force is the conformational change induced by the ligand in the extracellular domain. The kinase domain of dimeric receptors is activated leading to transmembrane signaling.
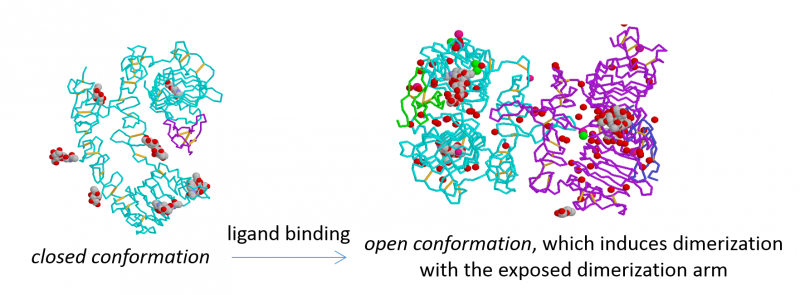
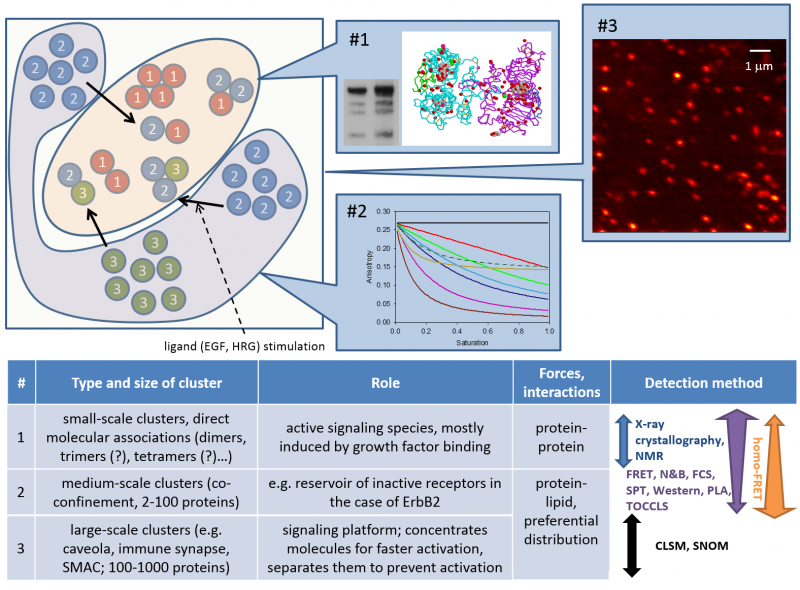

Our work has led to the following discoveries:
- ErbB and other membrane proteins generate hierarchical clusters, among which dimers are only the smallest types

- EGF receptor (ErbB1) and ErbB2 behave substantially differently regarding their large-scale clustering properties and their response to growth factor stimulation.

In our current research efforts we are investigating the role of the transmembrane and kinase domains in dimerization, and the constitutive and ligand-induced dimerization of the third member of the family, ErbB3.
Last update:
2023. 06. 21. 11:50