 The results of the research conducted at the Department of Biophysics and Cell Biology by Lőrinc Nagy, Marianna Mezősi-Csaplár, István Rebenku, György Vereb, and Árpád Szöőr help to describe more accurately the mechanisms of new types of gene-modified immune cell-based targeted cancer therapies. The effectiveness of chimeric antigen receptor (CAR) T cells in cancer therapy is limited because, in many cases, tumor cells become resistant to therapy by losing the expression of the therapeutic target. Universal CAR (UniCAR) T cells offer a solution to this problem by recognizing their molecular targets through adapter molecules, such as biotinylated antibodies, allowing for the simultaneous engagement of multiple targets by a single CAR T cell product.
The results of the research conducted at the Department of Biophysics and Cell Biology by Lőrinc Nagy, Marianna Mezősi-Csaplár, István Rebenku, György Vereb, and Árpád Szöőr help to describe more accurately the mechanisms of new types of gene-modified immune cell-based targeted cancer therapies. The effectiveness of chimeric antigen receptor (CAR) T cells in cancer therapy is limited because, in many cases, tumor cells become resistant to therapy by losing the expression of the therapeutic target. Universal CAR (UniCAR) T cells offer a solution to this problem by recognizing their molecular targets through adapter molecules, such as biotinylated antibodies, allowing for the simultaneous engagement of multiple targets by a single CAR T cell product.
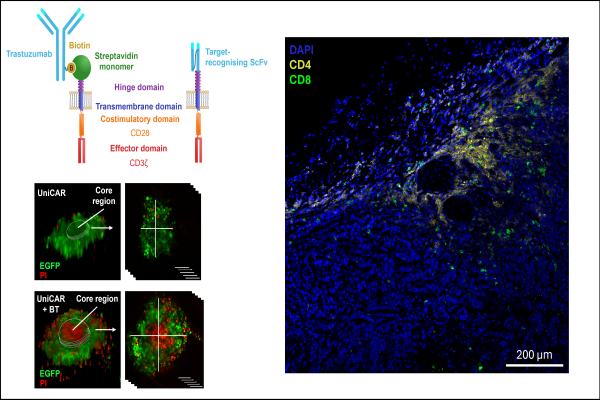
The research group has previously demonstrated that conventional CAR T cells are capable of penetrating the well-developed extracellular matrix (ECM) of tumors, which hinders the entry of therapeutic antibodies, thus providing protection for the tumor. In their current study, they examined whether UniCAR T cells, also directed by antibodies, are capable of tumor penetration similar to conventional CAR T cells. In the UniCAR construct used, a biotin-binding streptavidin 2 monomer (mSA2) served to bind a biotinylated trastuzumab (BT) antibody and through it, the (indirect) recognition of the HER2 target.
BT-targeted UniCAR T cells showed efficient and specific immune reactions in the presence of both surface-bound and membrane-expressed HER2 targets, and effectively penetrated tumor spheroids inducing cell death even in their central region. In vivo experiments proved that under physiological conditions, UniCAR T cells promptly bind circulating BT antibodies and consequently target HER2-positive tumor xenografts. However, due to the presence of native HER2 and biotin, this construct showed side effects in the lungs.
Based on these findings, the use of adapter molecules similar to biotin but not expressed in the body could represent an optimal solution against antibody therapy-resistant tumors showing high antigen heterogeneity. However, it is of paramount importance that the UniCAR bind the adapter molecule with high affinity to ensure proper penetration into solid tumors - a feat that the adapter molecule in itself would not be able to do.